New Platform Announcement: Intravenous (IV) Bags
Product Portfolio Expansion
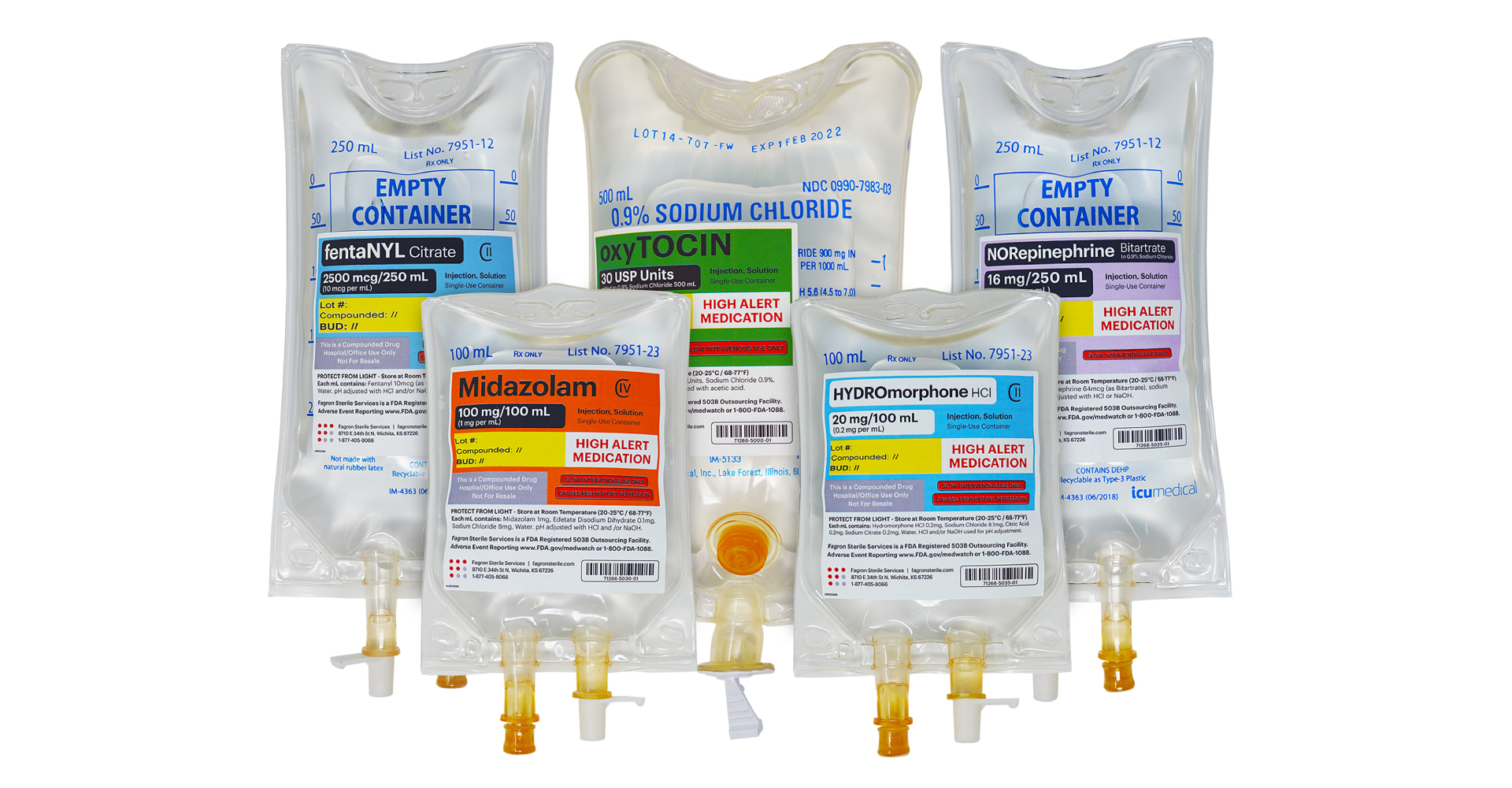
Fagron Sterile Services US is pleased to announce the continued expansion of its product portfolio in 2021. Introducing a new platform with multiple presentations available – Intravenous (IV) Bags. The new platform launches with multiple presentations of fentaNYL, HYDROmorphone, and midazolam. FSS will continue to expand IV Bag offerings to include oxyTOCIN, NORepinephrine, Vancomycin, Ketamine, PHENYLephrine, and others.
Product Platform Highlights:
- Individually overwrapped for extended beyond use dating (BUD) and product protection
- Multiple concentrations, volumes, and containers available
- Controlled medications are tamper-seal protected
- Committed supply agreements offered with 98% on-time delivery rate
- Expanded offerings throughout 2021
Download the IV Bag Launch Sheet.
New to FSS?
Click or tap "Request a consultation" to fill out a short form and knowledgeable and friendly representative will contact you directly to discuss your facility or health systems specific needs.
To view a current list of available products download a full product catalog today.
.png)
About Fagron Sterile Services
Fagron Sterile Services US (FSS), a top-tier DEA and FDA-registered 503B Outsourcing Provider, produces reliable supply of high-quality sterile medications with cGMP compliant operations for patient-focused healthcare facilities across North America.
FSSs team boasts operational and regulatory expertise in pharmaceutical manufacturing and repackaging, while leveraging industry-leading automation, advanced environmental monitoring, and sophisticated in-house quality testing labs. Batches are validated to support proper Beyond-Use-Dating (BUD) and only released after successful sterility, particulate matter, and potency testing are complete.
FSS offers a broad product portfolio across Operating Room Anesthesia, Ophthalmics, Pain Management and other Specialty Presentations, and holds Integrated Delivery Network (IDN) agreements within all major Group Purchasing Organizations (GPO).
Learn more specifically about our controlled substances.
