STERILE OUTSOURCING SERVICES
Sterile-to-Sterile, API-to-Sterile & Biologic compounding produced with industry-leading automation, quality & customer service
Reduce waste, cut down on the number of touch points in surgery preparation
Pre-filled, ready-to-administer solutions prepared in cGMP-compliant, DEA- and FDA-registered facilities inspected against 21 CFR 210 and 211 standards.

From the beginning, supporting your patient care needs
Ensuring the safety and well-being of patients is your top priority. As a trusted supplier, FSS helps you stay focused on delivering exceptional care.
Our high-quality sterile medications like oxyTOCIN and epidural solutions support growing families nationwide.

Support safety, improved efficiency, with high-quality ophthalmic products
For decades we've partnered with clinicians and are recognized among 503B outsourcing facilities as a leader in ophthalmics.
From combo eye drops to mitoMYcin and moxifloxacin, our innovative services support the unique needs of ophthalmic practices like yours.

Give your patients peace of mind with preservative-free corticosteroids
Helping you deliver high-quality care to your patients.
Unit-of-use options, prepared under stringent cGMP guidelines; bupivacaine, ropivacaine, fentanyl, hydromorphone, and more.

Innovation, from topical anesthesia to urology (mito-bladder) and dialysis
As a pioneer in 503B outsourcing, we offer a wide range of solutions designed to support safe and efficient patient care.
The first and only sterile L.E.T. Gel, a revolutionary clean container for repackaged Avastin®, mito-bladder syringes ready to support your USP <800> compliance, and more.

Together we create the future of personalizing medicine.
Over the past 34 years, Fagron has earned the trust of pharmacists in communities across more than 30 countries by prioritizing transparency and delivering high-quality medications supporting patient care.
Fagron Sterile Services US (FSS) is a DEA and FDA-registered and inspected supplier, providing a broad portfolio of high-quality sterile medication and expertise in pharmaceutical manufacturing, repackaging, patient safety, regulatory guidance and pharmacy.
268K+ ft²
Capacity across 3 state-of-the art operational facilities
100%
of batches tested for sterility, particulate matter and potency to support patient safety
Diverse product portfolio
Supporting providers and the patients you serve across the continuum of care
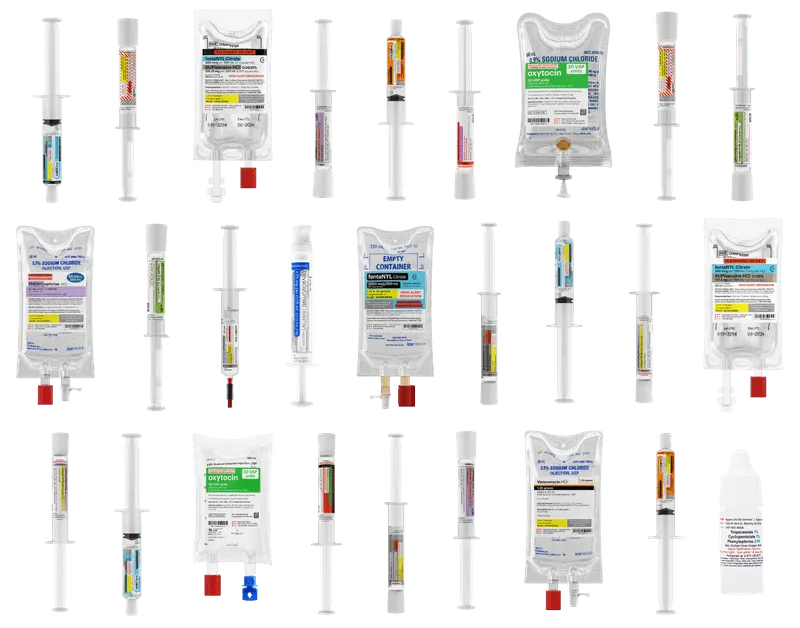
Instant ship program
Medications when you need them the most, in-stock, ready to ship

Get started in 4 easy steps
Trusted by thousands of healthcare providers nationwide
Supporting millions
One of the things that I appreciate the most about FSS is their large range of products."
Locations across the US



Speak with your personal representative today
Service is in our name and we can’t wait to partner with your patient-focused healthcare facility. Schedule a free consultation today to discuss your 503B outsourcing needs.
Globally and vertically integrated operations
Fagron’s 4k+ employees serve 30+ countries from 70+ facilities
Proactively supporting supply chain resiliency
Fagron’s global team of 100 supply chain experts leverage 3k+ suppliers
Diverse portfolio across the continuum of care
FSS supports patient care from the delivery room to the O.R.
including clinics

