
DEXAmethasone sodium phosphate Injection Solution, PF, 4 mg/mL (8mg/2mL), in a 3 mL Vial
Together we create the future of personalizing medicine.
Over the past 34 years, Fagron has earned the trust of pharmacists in communities across more than 30 countries by prioritizing transparency and delivering high-quality medications supporting patient care.
Fagron Sterile Services US (FSS) is a DEA and FDA-registered and inspected supplier, providing a broad portfolio of high-quality sterile medication and expertise in pharmaceutical manufacturing, repackaging, patient safety, regulatory guidance and pharmacy.
268K+ ft²
Capacity across 3 state-of-the art operational facilities
100%
of batches tested for sterility, particulate matter and potency to support patient safety
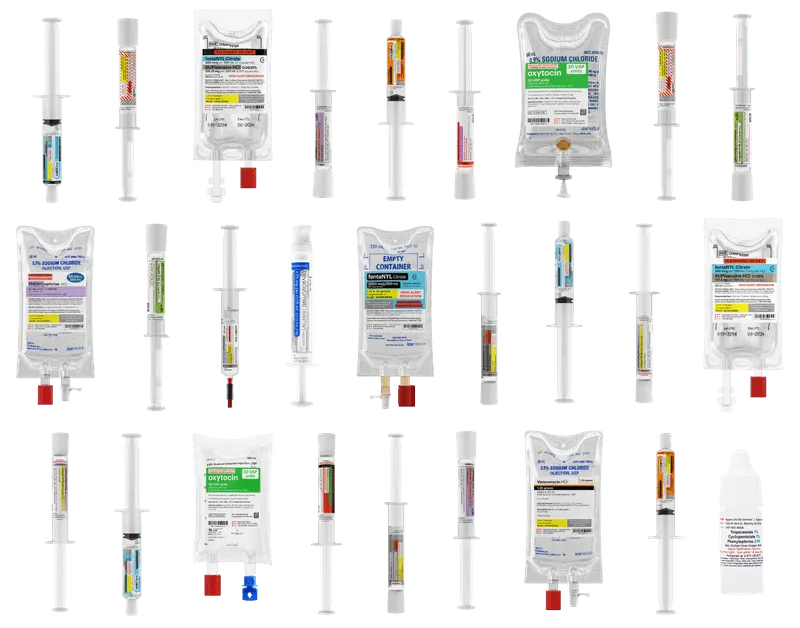
Diverse product portfolio
Supporting providers and the patients you serve across the continuum of care
Instant ship program
Medications when you need them the most, in-stock, ready to ship

Speak with your personal representative today
Service is in our name and we can’t wait to partner with your patient-focused healthcare facility. Schedule a free consultation today to discuss your 503B outsourcing needs.
Globally and vertically integrated operations
Fagron’s 4k+ employees serve 30+ countries from 70+ facilities
Proactively supporting supply chain resiliency
Fagron’s global team of 100 supply chain experts leverage 3k+ suppliers
Diverse portfolio across the continuum of care
FSS supports patient care from the delivery room to the O.R.
including clinics
