The first Sterile Topical L.E.T. Gel from a 503B outsourcing facility
LET Gel is a topical anesthetic consisting of Lidocaine, EPINEPHrine, and Tetracaine which is commonly used in conjunction with suturing patients in hospital emergency rooms, urgent care centers, pediatrician offices or by other qualified health care providers.

We developed a Topical LET Gel in response to the needs of healthcare providers, supporting clinicians in delivering effective pain management during wound care procedures
Sterile Topical L.E.T. Gel
FSS worked tirelessly to develop the first sterile version of a topical LET gel, listening to both customer needs and the FDA.
Sterile Topical LET Gel, from Fagron Sterile Services, is the only product of its kind on the market from a 503B outsourcing facility or compounding pharmacy.

FDA / DEA Registered & Inspected
Compliance with FDA, DEA, cGMP, GDP and cGLP regulations and guidance ensure our process is sound and supports patient safety
cGMP Compliant
Three decades of experience employing subject matter expertise with 21 CFR Parts 210 and 211 produces quality you can trust
ISO 5 environments
16 independent aseptic processing environments with dedicated HVAC systems, automation and depyrogenation technology
Committed Supply
The medication you want, when you need it — period. Talk with an Account Representative today about our Instant Ship program
100%Tested
All batches must successfully pass tests for sterility, particulate matter and potency prior to being released

Prepared in a world-class ISO 5 sterile environment
Subject matter experts from FSS’ Laboratory Services, Validation, Operations, and Quality collaborated to ensure the consistency of a sterile gel by employing a sophisticated, multi-step, process validation and following cGMP compliant operations at FSS’ state-of-the-art facilities, centrally located in Wichita, KS.
The manufacturing process of Sterile Topical LET Gel has been fully validated with Aseptic Process Simulation (Media Fill) and Stability Studies proving product efficacy throughout the life of its expiry date.
No refrigeration required
FSS recognizes that refrigerated storage space is often limited in many healthcare facilities. Our Topical LET Gel is formulated for convenient room temperature storage (20°C to 25°C), helping optimize space while maintaining product stability and readiness for use.
FSS dispenses Sterile Topical LET Gel in a convenient 3 mL unit dose syringe, available in 10-packs. As a 503B Outsourcing Facility, FSS can distribute LET Gel for shelf stock, no need for patient specific prescriptions.

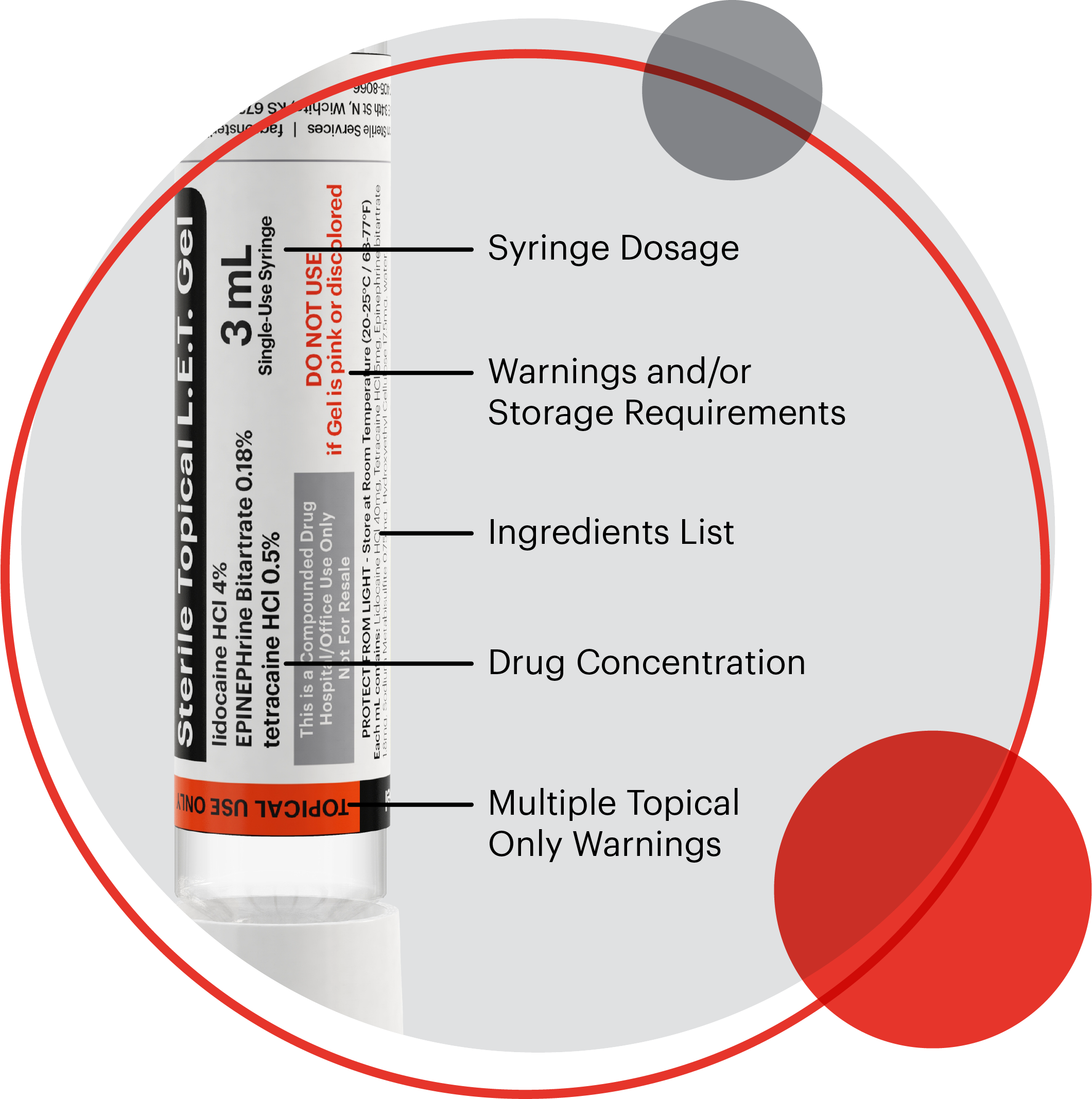
Advanced labeling with multiple route of administration safety warnings
Patient safety is critical. That is why every dose of FSS' Sterile Topical LET Gel is individually labeled and includes multiple safety warnings in different orientations to help minimize the risk of misadministration, including a "Topical Use Only" sticker over the tamper-evident cap.
FSS' product labels clearly display critical information:
• Presentation specifications
• Route of administration
• Storage requirements
• Expiration dating
• TALLman lettering
• Barcode
Together we create the future of personalizing medicine.
Over the past 34 years, Fagron has earned the trust of pharmacists in communities across more than 30 countries by prioritizing transparency and delivering high-quality medications supporting patient care.
Fagron Sterile Services US (FSS) is a DEA and FDA-registered and inspected supplier, providing a broad portfolio of high-quality sterile medication and expertise in pharmaceutical manufacturing, repackaging, patient safety, regulatory guidance and pharmacy.
268K+ ft²
Capacity across 3 state-of-the art operational facilities
100%
of batches tested for sterility, particulate matter and potency to support patient safety
Diverse product portfolio
Supporting providers and the patients you serve across the continuum of care
Instant ship program
Medications when you need them the most, in-stock, ready to ship

Talk to a representative to order today
FSS’ Sterile Topical LET Gel demonstrates a commitment to innovative solutions and partnership with customers to provide a reliable supply of high-quality medications for their patient-care needs.
Globally and vertically integrated operations
Fagron’s 4k+ employees serve 30+ countries from 70+ facilities
Proactively supporting supply chain resiliency
Fagron’s global team of 100 supply chain experts leverage 3k+ suppliers
Diverse portfolio across the continuum of care
FSS supports patient care from the delivery room to the O.R.
including clinics
